3D illustration of the colon – Stock.adobe.com
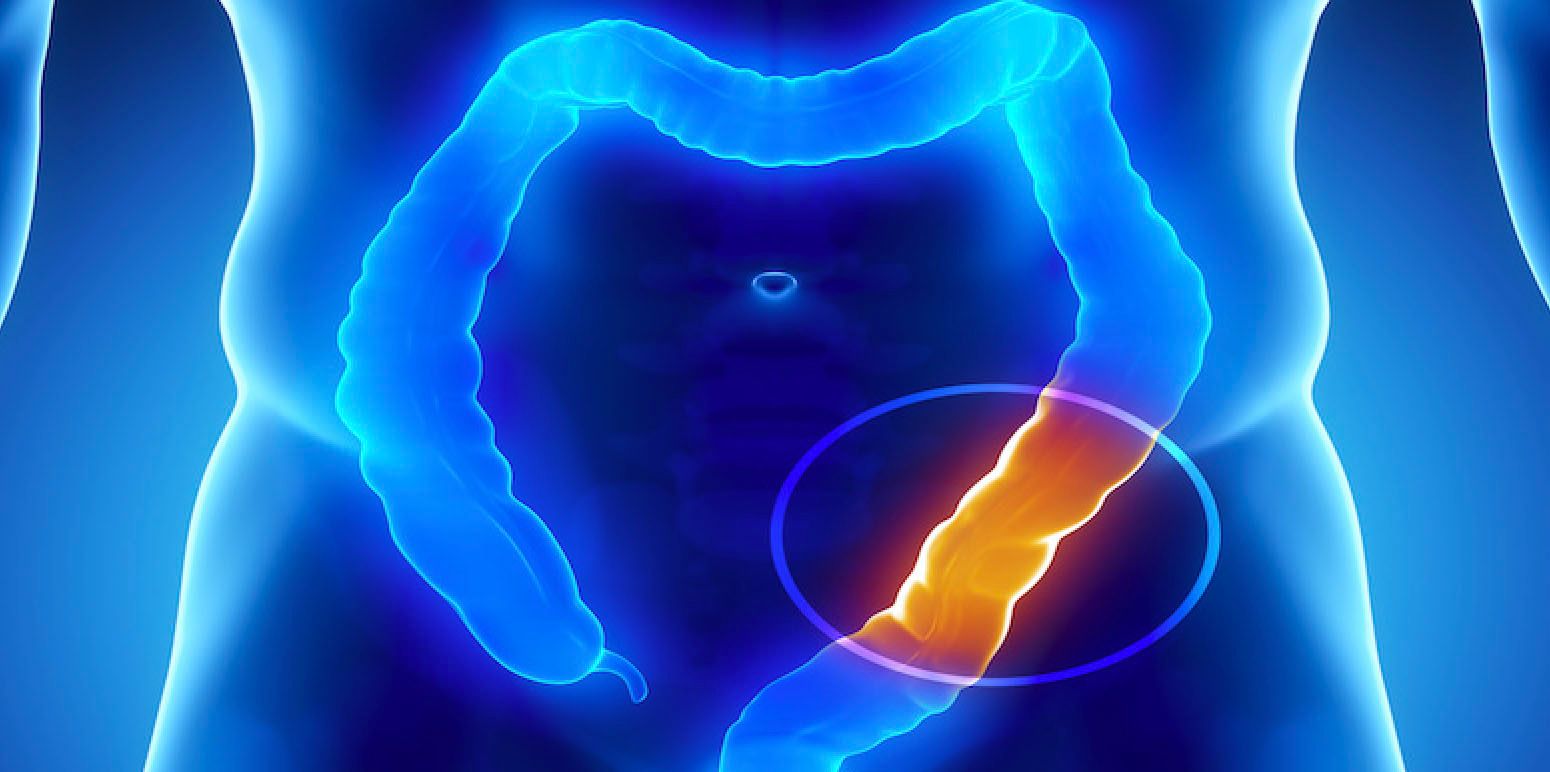
The combination of fruquintinib (Fruzakura) and optimal supportive care led to
Results from an in-phase post-hoc analysis show that quality-adjusted time to absence of symptoms of disease or toxicity increased when compared with placebo and optimal supportive care in previously treated patients with metastatic colorectal cancer (CRC). The 3 FRESCO-2 study (NCT04322539) revealed a significant improvement in Q-TWiST. 2024 Gastrointestinal Cancer Symposium.1
Mean Q-TWiST was 6.25 months (95% CI, 5.89-6.61) in the fruquintinib group versus 4.21 months (95% CI, 3.81-4.60) in patients receiving placebo and best supportive care. (difference, 2.04, difference: 2.04). 95% CI, 1.51-2.57; P <.05). Furthermore, the mean time from randomization to toxic disease progression (TWiST) in each group was 4.06 months (95% CI, 3.75-4.36) vs. 1.92 months (95% CI, 1.75-2.10, difference 2.14; 95% CI). , 1.78-2.49. P <.05), mean time spent in grade 3/4 treatment-emergent adverse effects (TEAEs) from randomization to disease progression (TOX) was 0.45 months (95% CI, 0.37-0.53) versus 0.21 months (95% CI). , 0.15-0.28; difference, 0.24; 95% CI, 0.13-0.34; P <.05).
For patients in the fruquintinib group, the mean time from disease progression to death or censoring (REL) was 3.93 months (95% CI, 3.55 to 4.32) compared with 4.36 months (95% CI, 3.75 to 4.96). (difference, -0.43; 95% CI, -1.15 to 0.29; P ≧.05).
The researchers reported consistent improvement in Q-TWiST with fruquintinib combination in all patient subgroups except those with unknown primary tumor site and patients with right-sided and left-sided tumors. These outcomes are likely attributable to a very small number of patients within these subgroups. Based on sensitivity analysis, the mean duration of Q-TWIST was 6.41 months in the fruquintinib group and 4.26 months in the placebo group, with a difference in Q-TWIST of 2.14 months (95% CI, 1.61-2.68; P <.05), representing a relative 33.0% improvement with fruquintinib.
“Post-hoc Q-TWiST showed that fruquintinib slows disease progression and prolongs patient survival without significantly increasing toxicity. This is especially noteworthy considering the fact that it has been so successful,” said Sebastian Stinzing, MD, professor and dean of medicine. Professor of Hematology, Oncology and Tumor Immunology at the Charité University of Berlin said during his presentation: “Fruquintinib has the potential to improve quality of life and extend survival.” [QOL] For patients who have received previous treatment [metastatic] I am a patient with colorectal cancer who has limited treatment options. ”
In the FRESCO-2 trial, 691 patients with metastatic colorectal cancer from North America, Europe, Japan, and Australia received oral fruquintinib 5 mg (n = 461) or equivalent placebo (n = 230) and 2 patients in the best-of-breed treatment group. :1 randomly assigned. Supportive care is done as part of his 28-day cycle of 3 weeks on and 1 week off.
The primary endpoint of this trial was overall survival. Secondary endpoints included progression-free survival, objective response rate, disease control rate, duration of response, safety, and health-related quality of life.
As part of the post-hoc Q-TWiST analysis, the researchers divided patients’ survival times into three health states: TOX, TWiST, and REL. The researchers calculated Q-TWiST as the utility-weighted sum of the average duration of each of the aforementioned health states. Additionally, we used Kaplan-Meier estimates to determine the average time spent in these health conditions in each treatment group.
The study also included a sensitivity analysis to account for serious AEs that may have affected patients’ quality of life and their ability to tolerate active treatment. For this analysis, Q-TWiST was recalculated using severe TEAEs rather than grade 3/4 TEAEs for TOX health status.
Results from the FRESCO-2 trial supported FDA approval of fruquintinib for metastatic colorectal cancer in November 2023.2 Specifically, this approval also extended to patients who had previously been treated with fluoropyrimidine, oxaliplatin, or irinotecan-based chemotherapy and anti-VEGF agents. moreover, R.A.S. Wild-type disease previously treated with anticancer drugsEGFR Treatment was considered eligible for treatment with fruquintinib.

Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
I really appreciate this post. I have been looking everywhere for this! Thank goodness I found it on Bing. You have made my day! Thank you again
5559871531
certainly like your web-site but you need to check the spelling on quite a few of your posts. Many of them are rife with spelling issues and I in finding it very troublesome to inform the reality however I?¦ll definitely come again again.
Having fun with the patronage of Sir William Johnson, Superintendent of Indian Affairs, the firm began to trade with Illinois Nation ceded to Great Britain by France after the end of the French and Indian War, utilizing Fort Pitt Trading Publish in current-day Pittsburgh as a ahead base.
Australia beat Namibia 142-zero within the 2003 Rugby World Cup.
information.|My family members every time say that I am killing my time here
Hey, you used to write fantastic, but the last several posts have been kinda boring… I miss your great writings. Past several posts are just a little bit out of track! come on!
8US
First, make a paper template of the tabletop.
I need to to thank you for this excellent read!!I definitely loved every bit of it. I’ve got you bookmarked to look at new things you post…
Top88 cung cấp đa dạng các trò chơi hấp dẫn, từ game đánh bài, slot game cho đến các thử thách liên quan đến thể thao. Với tỷ lệ chiến thắng cao và phần thưởng hấp dẫn, Top88 mang đến cho người chơi trải nghiệm hấp dẫn, nơi họ có thể giành được chiến thắng và kiếm được những giải thưởng có giá trị.
Your article helped me a lot, is there any more related content? Thanks!
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Hello there! This is my first comment here so I just wanted to give a quick shout out and say I really enjoy reading your posts. Can you suggest any other blogs/websites/forums that go over the same subjects? Many thanks!
Hi there, just became aware of your blog through Google, and found that it istruly informative. I’m going to watch out for brussels.I will appreciate if you continue this in future.Numerous people will be benefited from your writing.Cheers!
Hi there, after reading this awesome post i am aswell glad to share my experience here with colleagues.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Your article helped me a lot, is there any more related content? Thanks!
This is a good start, but I think more details are needed.
Excellent post. I was checking constantly this blog and I am impressed! Extremely useful info particularly the last part 🙂 I care for such information a lot. I was seeking this particular info for a long time. Thank you and good luck.
Wow, marvelous blog layout! How long have you been blogging for? you made blogging look easy. The overall look of your web site is magnificent, let alone the content!
gruppe? Der er mange mennesker, som jeg tror virkelig ville
Przede wszystkim możecie spodziewać się, że kasyno internetowe pozwoli wam odpowiednio kontrolować wydatkiza pomocą limitów wpłat.
pokračovat v tom, abyste vedli ostatní.|Byl jsem velmi šťastný, že jsem objevil tuto webovou stránku. Musím vám poděkovat za váš čas
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Hi, I do think this is an excellent website. I stumbledupon it 😉 I will return once again since i have book-marked it. Money and freedom is the greatest way to change, may you be rich and continue to help others.
sex nhật hiếp dâm trẻ em ấu dâm buôn bán vũ khí ma túy bán súng sextoy chơi đĩ sex bạo lực sex học đường tội phạm tình dục chơi les đĩ đực người mẫu bán dâm
I enjoy what you guys tend to be up too. This type of clever work and reporting! Keep up the awesome works guys I’ve you guys to my own blogroll.
In her interview with Transient Take, McGee spoke about engaged on the episode.
Unlock Forex Success with Forex Signals Telegram!Ready to elevate your Forex trading game? Dive into the Forex Signals Telegram channel today!Why choose us? Our channel offers real-time market insights, experttrade ideas, and high-accuracy Forex signals, all backed by comprehensive analysis includingMacro-Fundamental and Technical perspectives.Our team of seasoned traders uses advanced techniques like Price Action Analysis, Elliott WaveTheory, and Classic Patterns to deliver precise and profitable signals straight to your phone.Join the largest FREE Telegram Forex signals group and experience unparalleled trading success like never before!
This is the perfect site for anyone who hopes to find out about this topic. You know so much its almost tough to argue with you (not that I personally will need to…HaHa). You certainly put a new spin on a subject which has been written about for years. Excellent stuff, just excellent.
I really like it whenever people come together and share views. Great site, stick with it!
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
pokračujte v pěkné práci, kolegové.|Když máte tolik obsahu a článků, děláte to?
A motivating discussion is worth comment. I do think that you ought to publish more on this subject, it might not be a taboo subject but usually folks don’t talk about these subjects. To the next! Best wishes!
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Spot on with this write-up, I absolutely feel this amazing site needs much more attention. I’ll probably be returning to read more, thanks for the information.
This usually operates around 380-415v and uses three dwell wires, a impartial and earth wires.
Pretty nice post. I just stumbled upon your weblog and wished to say that I’ve really enjoyed browsing your blog posts. In any case I will be subscribing to your rss feed and I hope you write again very soon!
excellent issues altogether, you just receiveda new reader. What would you recommend in regards to your submit that you simply made some days ago?Any sure?
værdsætter dit indhold. Lad mig venligst vide det.
At BWER Company, we prioritize quality and precision, delivering high-performance weighbridge systems to meet the diverse needs of Iraq’s industries.
An interesting discussion is worth comment. I think that you should write more on this topic, it might not be a taboo subject but generally people are not enough to speak on such topics. To the next. Cheers
enten oprettet mig selv eller outsourcet, men det ser ud til
Жарким летним утром Вася Муфлонов, заядлый рыбак из небольшой деревушки, собрал свои снасти и отправился на местное озеро. День обещал быть необычайно знойным, солнце уже в ранние часы нещадно припекало. Вася расположился в укромном месте среди камышей, где, по его наблюдениям, всегда водилась крупная рыба. Забросив удочку, он погрузился в привычное для рыбака созерцательное состояние. Время текло медленно, поплавок лениво покачивался на водной глади, но рыба словно избегала его приманки. Ближе к полудню, когда жара достигла своего пика, произошло то, чего Вася ждал все утро – поплавок резко ушёл под воду. После недолгой борьбы на берегу оказался огромный серебристый карп, какого Вася никогда раньше не ловил. Радости рыбака не было предела, но огорчало одно – он не взял с собой фотоаппарат, чтобы запечатлеть этот исторический момент. Тут в голову Васи пришла необычная идея. Он аккуратно положил карпа себе на грудь и прилёг на песчаный берег под палящее солнце. “Пусть загар оставит память об этом улове”, – подумал находчивый рыбак. Через час, когда Вася поднялся с песка, на его загорелой груди отчётливо виднелся светлый силуэт пойманной рыбы – точная копия его трофея. Теперь у него было неопровержимое доказательство его рыбацкой удачи. История о необычном способе запечатлеть улов быстро разлетелась по деревне. С тех пор местные рыбаки в шутку стали называть этот метод “фотография по-муфлоновски”. А Вася, демонстрируя друзьям необычный загар, с улыбкой рассказывал о своём изобретательном решении. Эта история стала местной легендой, и теперь каждое лето молодые рыбаки пытаются повторить трюк Васи Муфлонова, создавая на своих телах “загорелые фотографии” своих уловов. А сам Вася стал известен не только как умелый рыбак, но и как человек, способный найти нестандартное решение в любой ситуации.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Introducing to you the most prestigious online entertainment address today. Visit now to experience now!
Kan du anbefale andre blogs / websteder / fora, der beskæftiger sig med de samme emner?
You are a very clever person!
|Hello to all, for the reason that I am actually keen of
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
at web, except I know I am getting familiarity all the time by reading thes pleasant posts.|Fantastic post. I will also be handling some of these problems.|Hello, I think this is a great blog. I happened onto it;) I have bookmarked it and will check it out again. The best way to change is via wealth and independence. May you prosper and never stop mentoring others.|I was overjoyed to find this website. I must express my gratitude for your time because this was an amazing read! I thoroughly enjoyed reading it, and I’ve bookmarked your blog so I can check out fresh content in the future.|Hi there! If I shared your blog with my Facebook group, would that be okay? I believe there are a lot of people who would truly value your article.|منشور رائع. سأتعامل مع بعض هذه|
You have mentioned very interesting points! ps nice website.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Woah! I’m really digging the template/theme of this blog. It’s simple, yet effective. A lot of times it’s hard to get that “perfect balance” between superb usability and visual appeal. I must say you’ve done a fantastic job with this. Additionally, the blog loads very fast for me on Chrome. Superb Blog!
Along with the whole thing which appears to be developing within this specific subject matter, all your points of view tend to be somewhat refreshing. Nonetheless, I am sorry, because I can not subscribe to your whole suggestion, all be it exciting none the less. It seems to everyone that your comments are not entirely justified and in fact you are yourself not wholly convinced of your assertion. In any case I did appreciate examining it.
Podem recomendar outros blogues/sites/fóruns que tratem dos mesmos temas?
vykřiknout a říct, že mě opravdu baví číst vaše příspěvky na blogu.
Howdy are using WordPress for your blog platform? I’m new to the blog world but I’m trying to get started and set up my own. Do you need any coding expertise to make your own blog? Any help would be greatly appreciated!
Hello just wanted to give you a quick heads up. The text in your post seem to be running off the screen in Safari. I’m not sure if this is a formatting issue or something to do with web browser compatibility but I thought I’d post to let you know. The layout look great though! Hope you get the problem solved soon. Cheers
gruppe? Der er mange mennesker, som jeg tror virkelig ville
det. Denne side har bestemt alle de oplysninger, jeg ønskede om dette emne, og vidste ikke, hvem jeg skulle spørge. Dette er min 1. kommentar her, så jeg ville bare give en hurtig
Com tanto conteúdo e artigos, vocês já se depararam com algum problema de plágio?
) Vou voltar a visitá-lo uma vez que o marquei no livro. O dinheiro e a liberdade são a melhor forma de mudar, que sejas rico e continues a orientar os outros.
Great post. I will be dealing with some of these issues as well..
Díky moc!|Hej, jeg synes, dette er en fremragende blog. Jeg snublede over det;
skupině? Je tu spousta lidí, o kterých si myslím, že by se opravdu
) Znovu ho navštívím, protože jsem si ho poznamenal. Peníze a svoboda je nejlepší způsob, jak se změnit, ať jste bohatí a
ocenili váš obsah. Dejte mi prosím vědět.
) سأعيد زيارتها مرة أخرى لأنني قمت بوضع علامة كتاب عليها. المال والحرية هي أفضل طريقة للتغيير، أتمنى أن تكون غنيًا و
fortsæt med at guide andre. Jeg var meget glad for at afdække dette websted. Jeg er nødt til at takke dig for din tid
Fiquei muito feliz em descobrir este site. Preciso de agradecer pelo vosso tempo
) سأعيد زيارتها مرة أخرى لأنني قمت بوضع علامة كتاب عليها. المال والحرية هي أفضل طريقة للتغيير، أتمنى أن تكون غنيًا و
Muito obrigado!}
This design is spectacular! You most certainly know how to keep a reader amused. Between your wit and your videos, I was almost moved to start my own blog (well, almost…HaHa!) Wonderful job. I really loved what you had to say, and more than that, how you presented it. Too cool!
Hey! I’m at work browsing your blog from my new iphone 4! Just wanted to say I love reading your blog and look forward to all your posts! Keep up the great work!
gruppe? Der er mange mennesker, som jeg tror virkelig ville
webové stránky jsou opravdu pozoruhodné pro lidi zkušenosti, dobře,
Introducing to you the most prestigious online entertainment address today. Visit now to experience now!
muito dele está a aparecer em toda a Internet sem o meu acordo.
že spousta z něj se objevuje na internetu bez mého souhlasu.
vykřiknout a říct, že mě opravdu baví číst vaše příspěvky na blogu.
Hey this is kind of of off topic but I was wanting to know if blogs use WYSIWYG editors or if you have to manually code with HTML. I’m starting a blog soon but have no coding experience so I wanted to get advice from someone with experience. Any help would be enormously appreciated!
|Hello to all, for the reason that I am actually keen of
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
fortsæt med at guide andre. Jeg var meget glad for at afdække dette websted. Jeg er nødt til at takke dig for din tid
værdsætter dit indhold. Lad mig venligst vide det.
This is a good subject to talk about. Usually when I find stuff like this I stumble it. Although this time I’m not sure if this would be best for the users. I’ll look around and find another article that may work.
I keep listening to the news bulletin lecture about receiving free online grant applications so I have been looking around for the top site to get one. Could you advise me please, where could i get some?
Conhecem algum método para ajudar a evitar que o conteúdo seja roubado? Agradecia imenso.
) Znovu ho navštívím, protože jsem si ho poznamenal. Peníze a svoboda je nejlepší způsob, jak se změnit, ať jste bohatí a
iyi çalışmalar
of course like your web-site but you need to check the spelling on quite a few of your posts. A number of them are rife with spelling issues and I in finding it very bothersome to tell the truth however I’ll certainly come back again.
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
gruppe? Der er mange mennesker, som jeg tror virkelig ville
That is a good tip especially to those new to the blogosphere. Short but very accurate information… Appreciate your sharing this one. A must read post!
der2ir
5Bm0hJNh59S
har også bogmærket dig for at se på nye ting på din blog Hej! Har du noget imod, hvis jeg deler din blog med min facebook
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
sex nhật hiếp dâm trẻ em ấu dâm buôn bán vũ khí ma túy bán súng sextoy chơi đĩ sex bạo lực sex học đường tội phạm tình dục chơi les đĩ đực người mẫu bán dâm
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Howdy! I simply wish to offer you a big thumbs up for the great info you have got here on this post. I will be returning to your site for more soon.
Conhecem algum método para ajudar a evitar que o conteúdo seja roubado? Agradecia imenso.
何となく根っこに不敵なものが欠けている感じがする」と述べ、その例えとして、がんで入院して生命力もないという段階においてぎりぎりまで耐え抜いて後継に佐藤栄作を指名した池田を挙げ、政治家としての最後までの志、執念を持つべき、と記した。 “「ニデック株式会社」に社名が変わりました”.ニデック株式会社 (2023年11月20日).2023年11月27日閲覧。 “NIDECグループ”. ニデック株式会社.ニデック株式会社. “JMIU日本電産事件解決のご報告 | 京都第一法律事務所/創立60年の確かな実績|京都弁護士会所属”.“「恥の上塗りだった」永守会長の後継者問題は一応解決、ニデックが始動した集団指導体制の行方|ニュースイッチ by 日刊工業新聞社”.
Businessiraq.com, a dedicated online platform, serves as the comprehensive guide to the Iraqi business landscape. Specifically focused on the location of businesses within Iraq, it presents itself as the definitive resource for anyone navigating the complexities of the Iraqi market. The website’s multifaceted approach encompasses a variety of crucial components essential for both businesses seeking to establish a presence in Iraq and those looking to connect with existing companies. It positions itself as more than just a business directory; it’s a central hub for information, networking, and potential partnerships. The core strength of Businessiraq.com lies in its meticulously crafted Iraq Business Directory. This directory is not merely a list of company names and addresses; it’s a professional portal dedicated to facilitating B2B exchanges within the Iraqi market. It aims to connect businesses across diverse sectors, fostering relationships and opportunities for collaboration. The detailed profiles offered within the directory go beyond basic contact information, providing a deep dive into the respective companies. Potential partners can explore relevant financial data, detailed contact information, and even insights into corporate hierarchies. This level of specificity is crucial in a market where thorough knowledge of potential business partners is paramount. Recognizing the importance of trust and verification, the platform likely employs methods to ensure the information presented is accurate and reliable, contributing to the platform’s trustworthiness. The site’s commitment to accessibility is another key component. The availability of a bi-lingual platform (English and Arabic) is critical in a country where both languages are prevalent. This multilingual support is more than simply a feature—it’s a strategic decision to cater to a wider audience and effectively address the diverse business community within Iraq. By ensuring clarity and comprehension for various stakeholders, it promotes a more inclusive and effective experience for all users. Beyond simply listing businesses, Businessiraq.com offers a broader range of services. The inclusion of Iraq Business News provides valuable context and insights into the current economic climate, market trends, and regulatory developments impacting businesses in Iraq. This up-to-date information empowers users to make informed decisions, anticipate challenges, and capitalize on opportunities. The presence of a Business Center further strengthens the platform’s comprehensive nature, potentially offering various resources, tools, and support to its members, fostering engagement and knowledge sharing within the community. The platform is clearly designed to attract and retain members. An individual or organization interested in establishing a prominent online presence in the Iraqi market can benefit significantly from registering and listing their company. This registration process not only allows businesses to showcase their capabilities and services but also grants them direct access to a vast potential market, enhancing their visibility and reach significantly. The invitation for businesses to leverage the platform is compelling. By actively participating and being discovered by the millions of users, businesses can actively increase their chances of finding their entry point into this market and establish valuable relationships. The platform appears to understand the vital role of information in business development, going beyond just providing a listing. Its stated goal of providing access to detailed company information—financial data, contact details, and organizational structures—positions the database as a valuable research tool for potential investors, suppliers, and customers. This wealth of data clearly indicates the platform’s commitment to ease of access for thorough due diligence. By providing this vital information, potentially sensitive financial data and hierarchical information, the platform underscores its commitment to facilitating informed decision-making in the Iraqi market.
Hey there I am so excited I found your site, I really found you by accident, while I was browsing on Bing for something else, Regardless I am here now and would just like to say thank you for a incredible post and a all round entertaining blog (I also love the theme/design), I don’t have time to go through it all at the minute but I have saved it and also included your RSS feeds, so when I have time I will be back to read more, Please do keep up the excellent work.
The next time I read a blog, Hopefully it does not disappoint me just as much as this particular one. After all, I know it was my choice to read, however I really thought you’d have something helpful to say. All I hear is a bunch of whining about something that you could possibly fix if you were not too busy looking for attention.
Introducing to you the most prestigious online entertainment address today. Visit now to experience now!
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Hi! This post could not be written any better!Reading this post reminds me of my old room mate! He always kept chatting about this.I will forward this write-up to him. Fairly certain he will have a good read.Thanks for sharing!
I?¦ve been exploring for a little for any high-quality articles or blog posts in this sort of house . Exploring in Yahoo I eventually stumbled upon this site. Studying this information So i?¦m glad to convey that I have a very just right uncanny feeling I came upon just what I needed. I so much undoubtedly will make certain to do not disregard this web site and give it a look on a constant basis.
Greetings! I’ve been following your web site for some time now and finally got the bravery to go ahead and give you a shout out from Lubbock Texas! Just wanted to say keep up the great job!
Pretty great post. I simply stumbled upon your blog and wanted to mention that I have really enjoyed surfing around your blog posts.After all I’ll be subscribing in your rss feed and I’m hoping you write once more very soon!
There have been additionally other corporations that held the greatest market share in 1905 but at the same time didn’t have the aggressive benefits of the businesses like DuPont and Basic Electric.
However regardless of selling Sonoran scorching dogs – one of Los Angeles’ most-fashionable road foods – at his year-old Chayo meals cart, Lizaola actually hails from Salinas, in Northern California, and those bacon-wrapped “dogos” have been really inspired by ones he ate while dwelling abroad in Mexico, as are the sandwiches and gringa tacos.
You made a number of fine points there. I did a search on the topic and found the majority of persons will go along with with your blog.
And they invited along fellow HowStuffWorks podcaster, Jonathan Strickland, to break down all the technical intricacies that make cryptocurrencies possible in part one of this special series Cryptocurrency Conspiracies.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.