research design
This prospective cohort study was conducted in adults from July 2022 to February 2023. At the time of the research, China was strict against the new coronavirus infection, which is controlled by Omicron. [14, 15]is a variant of SARS-CoV-2, and all people underwent population-wide coronavirus screening with routine universal reverse transcription-polymerase chain reaction (RT-PCR) testing in hospital or in the community. All suspected coronavirus cases, even those with asymptomatic infections, were referred to local hospitals or large square shed hospitals for mandatory isolation. All suspected cases who underwent diagnostic procedures, including nasopharyngeal polymerase chain reaction (PCR) tests and chest X-rays to show pulmonary involvement, had been given a confirmed diagnosis of coronavirus infection by a doctor. Patients with COVID-19 will be included in the study according to inclusion or exclusion criteria as soon as the diagnosis of COVID-19 is confirmed. Gastroenteroscopy including endoscopic biopsy, blood draws (blood count, white blood cell count, CPR, PCT, IL-6, IgG and IgM antibody tests for viruses) and other tests (X-rays and ultrasound, regular urination) , regular flights) were required. To exclude other bacterial and viral infections of the whole body or parts of the body, or acute gastroenteritis (AG), which can cause gastrointestinal symptoms. The time lag between COVID-19 diagnosis and study participation is less than one day. All patients received the standard treatment national protocol without modification.
It consisted of two cohorts, case groups containing patients with COVID-19 infection who were consecutively recruited from Fangcai Hospital, a dedicated COVID-19 treatment center in Haikou, Hainan Province, China. The healthy control group included COVID-19 serologically negative screeners who underwent screening at our hospital during the same period. Participants were followed up with outpatient physical examinations or telephone calls at 1, 3, and 6 months using the validated her Rome III and Rome IV questionnaires and limited objective assessments. [1]. The follow-up period was over 6 months from July-August 2022 to February 2023. Data from the case group were compared with her 6-month follow-up data regarding the development of her FGID in a cohort of age- and sex-matched healthy subjects. In addition, subjects who met various diagnostic criteria for FGID in Rome at 6-month follow-up were given clinical indications to exclude some diseases such as gastroparesis and SIBO, as well as laboratory tests and He was advised to come to the clinic for further endoscopy. Clinical indicators include duration and frequency of diarrhea, stool consistency and presence of blood, history of vomiting, fever, crampy abdominal pain, and weight loss. The included COVID-19 patients and healthy controls were tested for COVID-19 infection after 3 and 6 months to exclude reinfection with COVID-19. The questionnaire also included items regarding comorbidities (hypertension or diabetes), anxiety, irregular eating, sleep quality, and regular exercise.
Inclusion criteria for the case group were: (1) persons between the ages of 18 and 85 years with a confirmed diagnosis of coronavirus of varying severity; [16, 17] (mild and moderate) using biochemical test data (e.g. COVID19-SARS-CoV-2 positive, anti-SARS-CoV-19 IgG and IgM negative) or computed tomography scan of the chest and observation of pulmonary involvement; (2 )No, a medical history with a clinically confirmed diagnosis of FGID, no gastrointestinal tumors, reflux esophagitis, ulcerative colitis or other gastrointestinal diseases, and no comorbidities (hypertension or diabetes). , no history of abdominal surgery, and no recurrence of baseline symptoms of FGID or gastrointestinal symptoms such as diarrhea, constipation, or abdominal pain. (3) Blood draws and other tests were required to rule out AG, bacterial and viral infections. (4) Normal findings of gastroscopy and other laboratory tests from a physical examination within the past 6 months were required. The healthy control group had no history of COVID-19 infection or FGID. Biochemical test data (e.g., COVID19-SARS-CoV-2 negative, anti-SARS-CoV-19 IgG and IgM negative) were required, and the remaining criteria were the same as for the case group.
The purpose of this study was explained in detail to all participants, and they were asked to fully understand and participate in this study based on their wishes. Additionally, written informed consent was obtained from each participant. Meanwhile, each participant provided a written or electronic informed consent form, and this study was approved by the Institutional Ethics Committee of the Second Hospital of Hainan Medical University (Reference number: LW2022270).
definition
RT-PCR was used to detect SARS-CoV-2 from subjects’ nasopharyngeal and oropharyngeal samples [18]. The diagnosis of IBS according to the Rome IV diagnostic criteria has changed significantly, and the new diagnostic criteria have increased the frequency needed to diagnose IBS, and the incidence of IBS has decreased. [1]. We used Rome III rather than the more recently described Rome IV criterion. This is because the latter is 50% less sensitive in diagnosing her IBS. [1].Other types of his FGID were diagnosed based on Rome IV criteria [1].
Indigestion refers to postprandial discomfort syndrome, which often manifests as a feeling of fullness or early satiety (inability to complete a normal meal) after a meal. Dyspepsia has the same meaning as functional dyspepsia and refers only to postprandial discomfort syndrome.
AG was defined as the presence of at least two of the following: (i) diarrhea, (ii) vomiting, (iii) fever, and (iv) stool cultures that isolated enteric pathogens. [6]. Stool samples from each coronavirus patient were examined under a microscope to detect pus cells, red blood cells, and parasites. All suspect stool samples were cultured for Vibrio cholerae, Salmonella enterica, Trichophyton rubrum, Campylobacter, and Aeromonas using standard techniques for identifying pathogenic bacterial strains.
Patients who show signs of organ failure (e.g., persistent oliguria, severe respiratory distress) or who require ventilator support or admission to an intensive care unit during treatment for COVID-19 were defined as having severe COVID-19 infection.The seriousness of the new coronavirus infection (COVID-19) [16, 17, 19] (i) severe (ventilator required), (ii) severe (oxygen required), (iii) moderate (pneumonia present but oxygen not required), and (iv) mild (upper respiratory tract symptoms only). People without symptoms at the time of COVID-19 diagnosis were classified as asymptomatic as a categorical variable (yes or no).
Irregular eating was defined as eating frequently (more than 5 times a month) and having irregular meal times. Pittsburgh Sleep Quality Index (PSQI) [20] It was used to investigate patients’ sleep quality. The total PSQI score ranges from 0 to 21, with 7 being the threshold for sleep quality problems, and high scores above 7 indicating poor sleep quality in the patient and vice versa. indicates that it is good. Regular physical activity is defined by the latest World Health Organization (WHO) Guidelines on Physical Activity and Sedentary Behavior 2020. [21]. Adults should do at least 150 to 300 minutes of moderate-intensity aerobic exercise, or at least 75 to 150 minutes of vigorous-intensity aerobic exercise, or an equivalent combination of moderate and vigorous intensity per week. Older adults should do at least 150 to 300 minutes of moderate-intensity aerobic exercise, or at least 75 to 150 minutes of vigorous-intensity aerobic exercise, or an equivalent combination of moderate-intensity and vigorous-intensity exercise per week .hamilton anxiety scale [22] It was used to assess the psychological state of the case group, and the possibility of anxiety was considered if the scale score exceeded 7.
statistical analysis
Sample size calculation
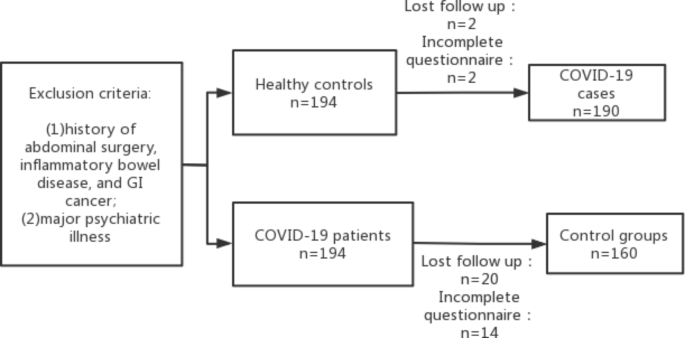
In this study, the sample size was calculated as 90% power and 99% confidence interval (two-tailed) based on previous studies. [12, 23, 24] It showed that the average incidence of FGID after acute gastroenteritis was approximately 21%, and a Chinese study showed that the incidence of PI-FGID in controls was 8.2%. [25]. The study required a total of 155 COVID-19 patients and 155 healthy controls, but because of the potential loss to follow-up of approximately 10% to 20%, 194 COVID-19 patients were required. Patients with coronavirus infection and 194 controls were enrolled. Four of the 194 COVID-19 patients and 34 of the 194 healthy controls were excluded because documentation was insufficient or lost during follow-up. Finally, 190 COVID-19 patients and 160 healthy controls were analyzed (Figure 1).
Data collection and analysis
SPSS (version 26; SPSS Inc, Chicago, IL) was used for all statistical analyses. Categorical data were expressed as percentages, and continuous data were expressed as mean ± standard deviation (SD) or median and range or interquartile range (IQR). Categorical variables were analyzed using the χ test with Yates correction where applicable. Parametric and nonparametric continuous data were analyzed using unpaired t-test and Mann-Whitney U test, respectively. Multivariate analysis was performed using stepwise logistic regression. A two-sided P value < 0.05 was considered significant.
